Mechanisms of cellular memory during normal growth and chronic pathological stress conditions
The Polycomb (PcG) and the Trithorax (TrxG) group proteins form the basis of a cellular memory system, which heritably maintains the transcriptional state of their target genes during development. Initially identified in Drosophila as regulators of homeotic genes, PcG/TrxG proteins were found to control a variety of target genes implicated in important biological processes like stem cell function, mammalian X chromosome inactivation and genomic imprinting in plants. PcG/TrxG proteins act as multisubunit complexes involved in the modulation of chromatin structure and covalent post-translational modifications of histones.
Developing new bioinformatics tools to assess PAR-CLIP data
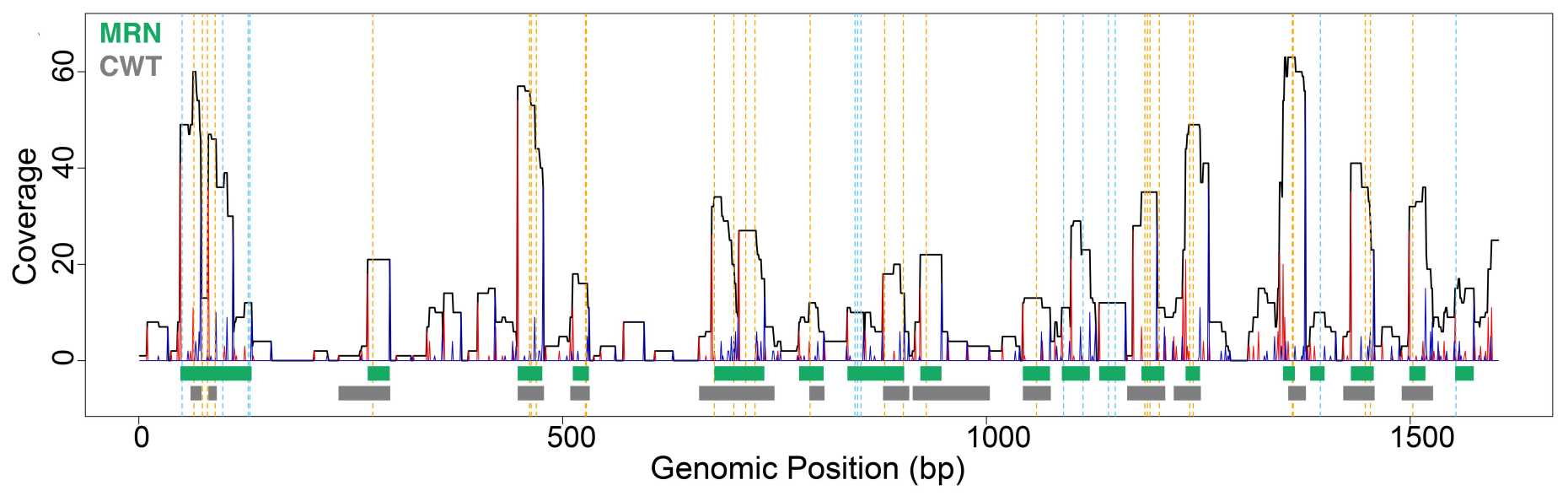
Many PcG proteins were found to interact with non-coding RNAs. The functional implications, however, of this partnership are still controversial. The group used the PAR-CLIP method to map the interactions of specific chromatin proteins with RNAs genome-wide. The method leaves an imprint in the RNA sequence at the protein binding site, which can be mapped and identified using next generation sequencing methods. A new algorithm wavClusteR was developed to reveal these interaction sites at high confidence.
Rapid degradation of proteins allows direct response read-outs
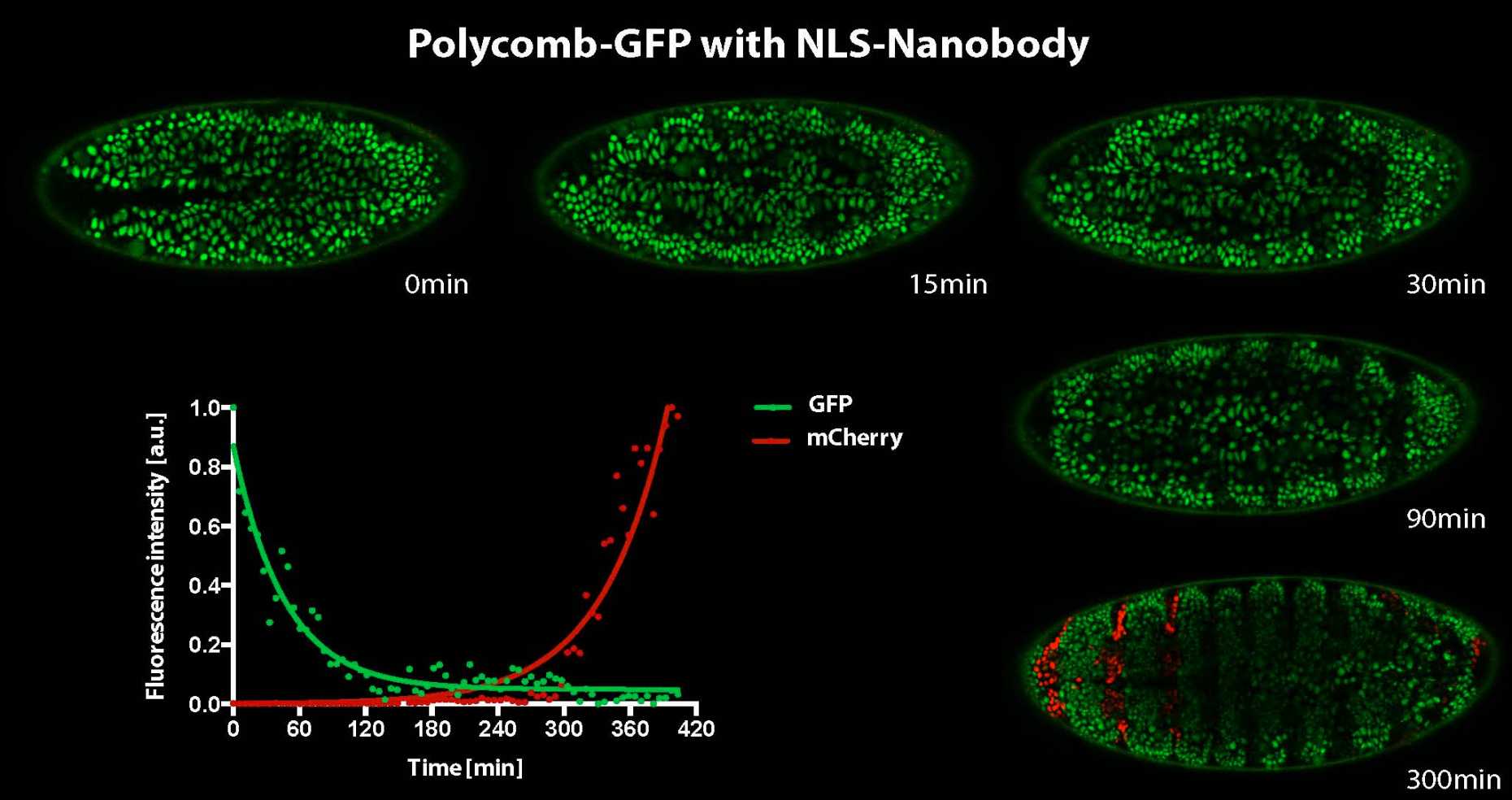
Even though epigenetic memory can be long lasting, the underlying structure of chromatin components appears to act in a highly dynamic manner in the nucleus. Depletion of chromatin factors using degron systems now allow us to analyze and directly dissect the consequences on gene expression in a highly time-resolved and cell cycle dependent manner.
Reprogramming cellular memory by environmental signals
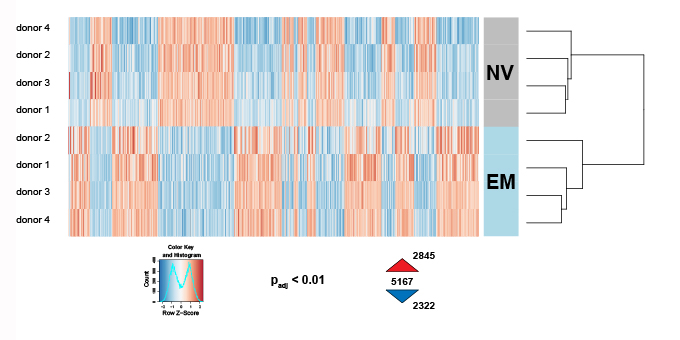
Unlike genetic information, epigenetic states can be modulated and are reversible. This has been particularly well demonstrated with somatic nuclear reprogramming or with the induced pluripotent stem cell technology. While certain epigenetic states have to be highly stable, like those defining the mammalian inactive X chromosome, others have to change according to cues received by the cell (e.g. metabolic or inflammatory signals). Hence, it can be expected that chronic inflammatory stress conditions, if maintained by epigenetic mechanisms, eventually can be reverted. The group examined whether particular environmental cues can also reverse adverse epigenetic information generated by metabolic and inflammatory stress that contribute to syndromes like diabetes and obesity in mice and humans. In collaboration with the groups of Christoph Hess and Marc Donath (University Hospital Basel) the group explored how a pathologic activation of the immune system contributes towards the development of metabolic syndromes. The project, funded by a Sinergia grant from the Swiss National Science Foundation, investigated how acute vs. chronic nutritional stress impacts the metabolic function and the epigenetic landscape of innate and adaptive immune cells.