Systems Immunology
Immune repertoire diversity
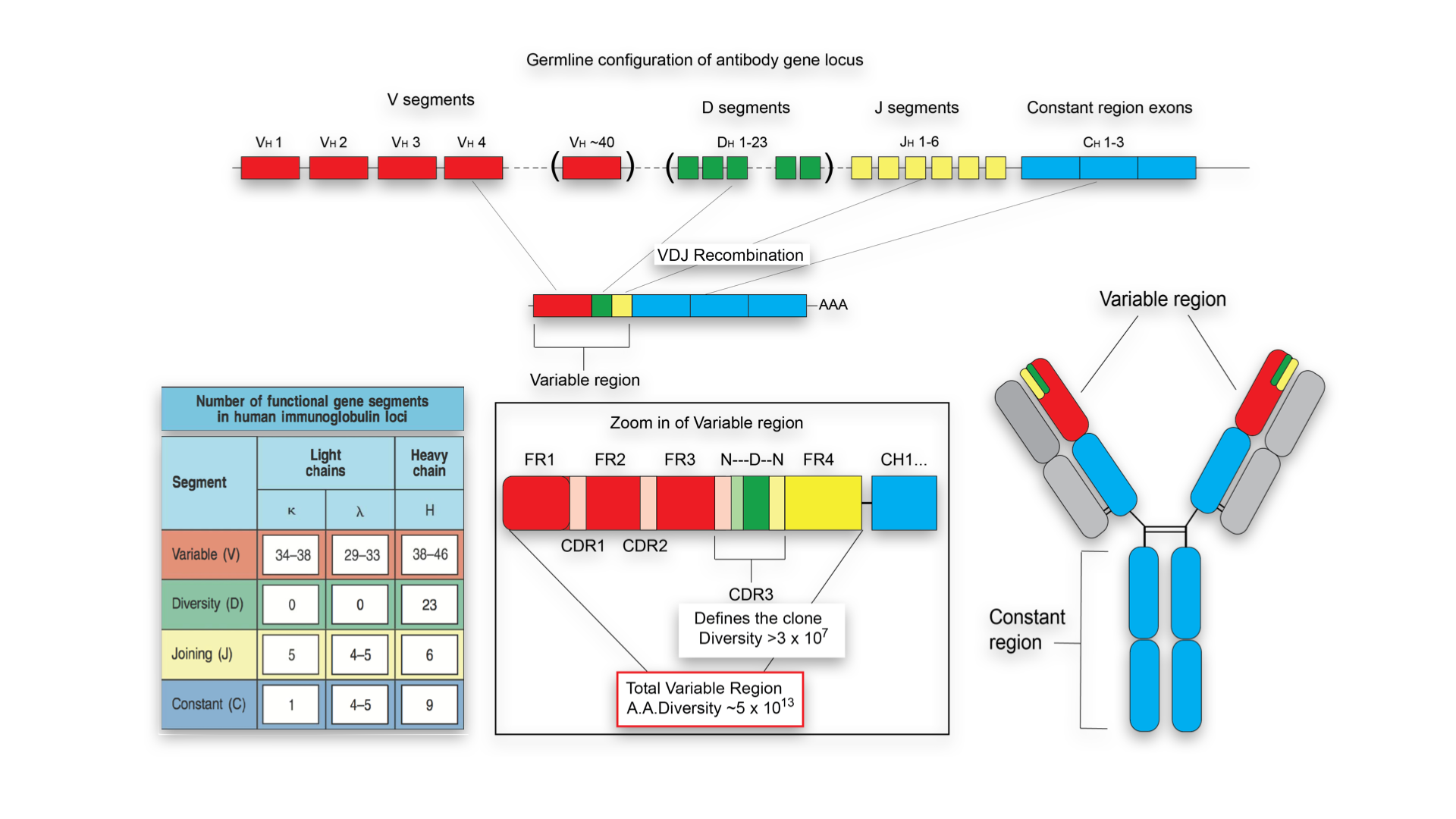
Every time an antigen, whether natural (pathogen) or recombinant (vaccine), encounters the immune system it leaves behind a molecular imprint that encodes valuable information. This antigenic fingerprint is encoded within the immune response, more specifically in the genetic and molecular identities – the antibody and T cell receptor repertoires. For example, primary antibody diversity in B cells is generated by the recombination of non-contiguous germline gene segments, variable (V), diversity (D), and joining (J) gene segments; this process of V(D)J recombination occurs during B lymphocyte development in bone marrow. Additional antibody diversification is provided by combinatorial pairing and somatic hypermutation of the variable heavy (VH) and variable light (VL) chains. All of these diversity elements combine to generate an antibody repertoire that is estimated to be >1013 in humans.
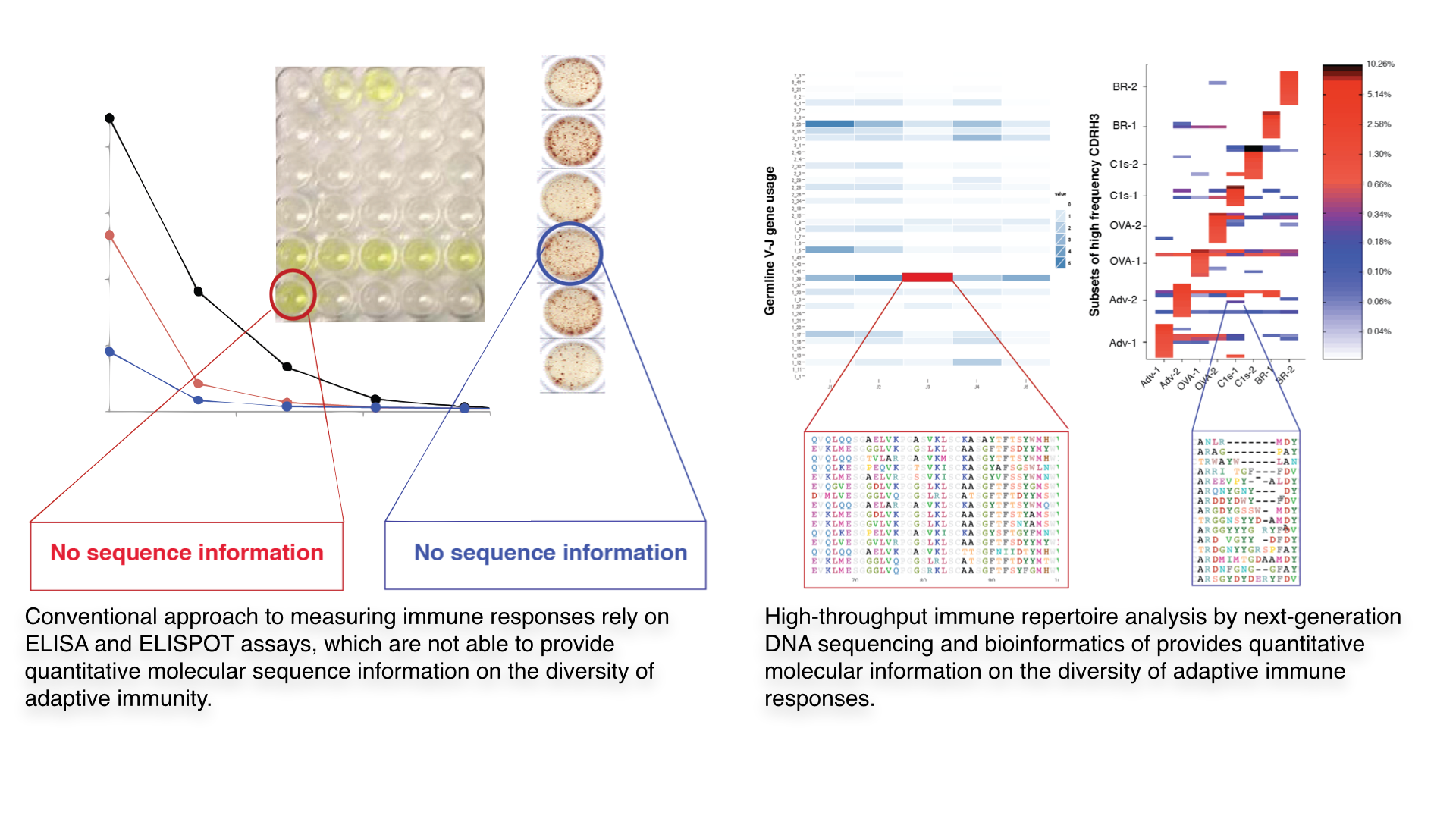
The conventional approach for measuring immune responses relies on cursory biological measurements, primarily antibody binding studies with serum (titers) or antigen-reactive lymphocyte assays (ELISPOT). These measurements provide a global view of adaptive immunity, however they are amenable to significant variation; furthermore they are lacking in their ability to quantitatively define the molecular composition of a polyclonal (diverse) immune response, which plays an indispensable role in pathogen defense. Therefore to truly understand and exploit the immune system’s capability for providing pathogenic defense requires moving beyond simple rudimentary measurements, in other words transitioning from analog to digital analysis of immune responses.
High-throughput immune repertoire analysis
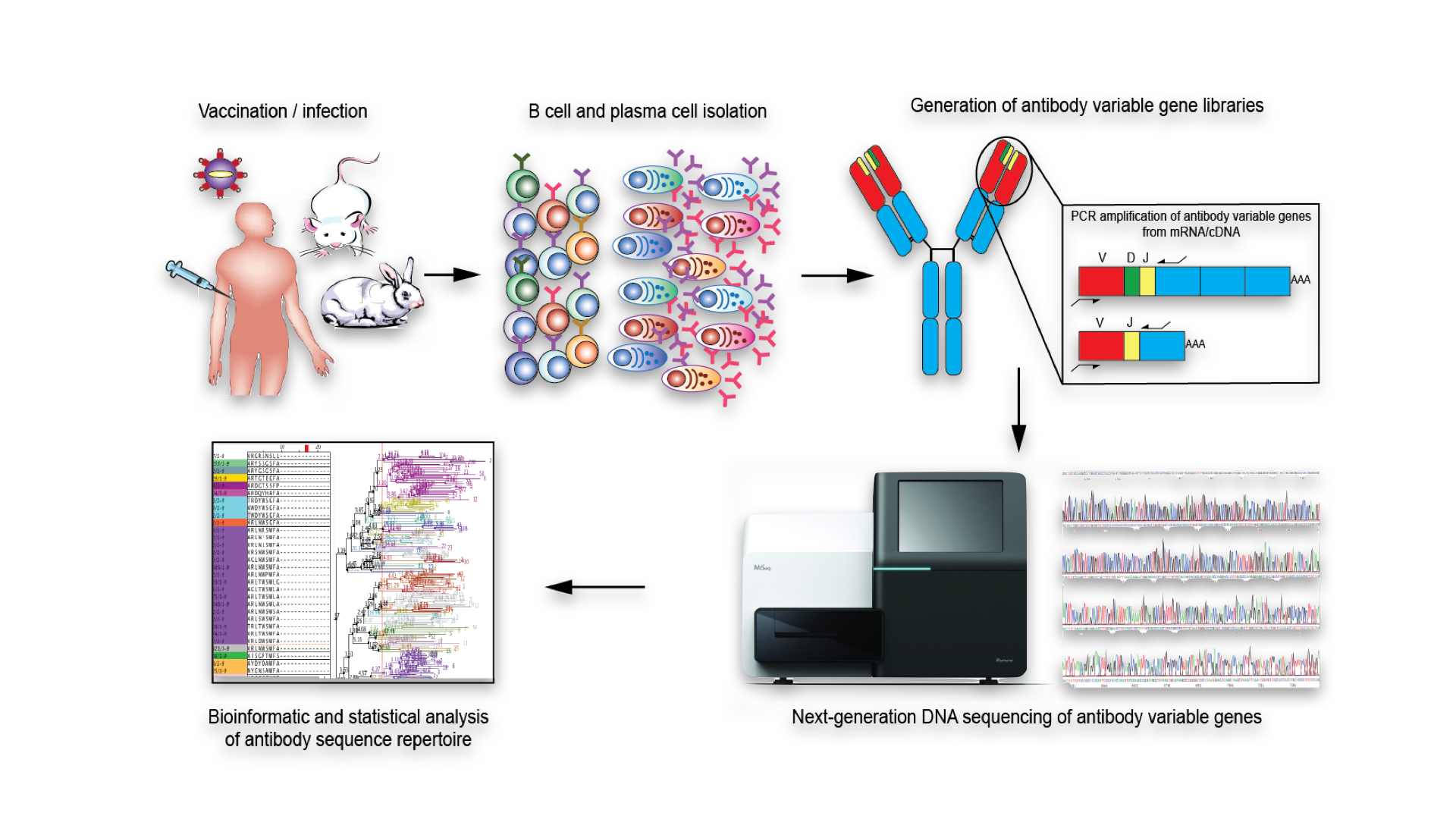
High-throughput immune repertoire analysis provides a quantitative approach towards answering basic questions related to diversity, development, and evolution of adaptive immune responses. Our lab is aims to establish a core-competency in this exciting new area of systems immunology, which provides a quantitative molecular profile of immune responses. High-throughput immune repertoire analysis is accomplished by combining next-generation DNA sequencing of immune cell receptor genes (BCRs/antibodies or T cell receptors) coupled to bioinformatic analysis. This approach is capable of quantifying the vast molecular diversity of an immune response, which is at the heart of the immune system’s physiological function of pathogen defense. Furthermore, we integrate immune repertoire analysis with biomolecular and cellular engineering in order to reconstruct the components of the immune response that are responsible for pathogen protection, which can be exploited in applications such as immunosignature diagnostics, vaccine profiling, and monoclonal antibody discovery and engineering.
Methods development in high-throughput antibody sequencing
We have built a comprehensive pipeline for both experimental and computational methods in antibody high- throughout sequencing. These include library preparation of antibody amplicons, quantitative statistical assessment of clonal repertoires, and rapid germline and frequency annotation.
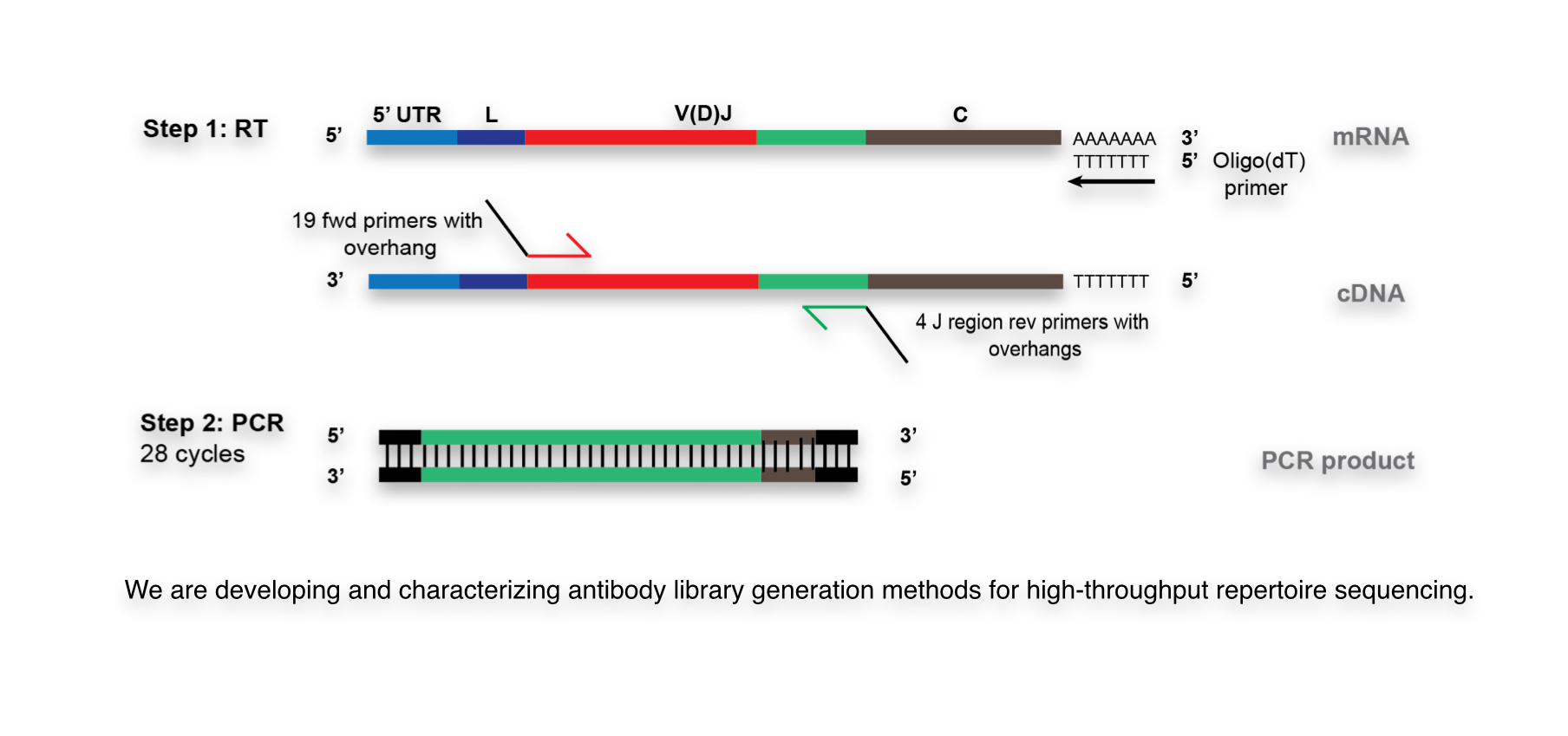
For example, we have developed several PCR-based approaches and compared them quantitatively in order to determine the impact of library preparation on antibody clonal repertoire diversity and distribution (U. Menzel, V. Greiff et al., PLoS ONE, 2014). Furthermore, we have also quantitatively evaluated the impact of undersampling on the robustness and reproducibility of antibody HTS datasets. By using triplicate samples we were able to assess how clonal frequencies were affected by undersampling; we found that there was extremely high correlation between the triplicate datasets within a large percentage of the repertoire, indicating that undersampling is playing a minor role (V. Greiff, U. Menzel et al., Manuscript In Review).
It has recently been demonstrated that HTS from multiplexed PCR library preparation can lead to significant biases, thus altering the accurate quantification of repertoire distribution and diversity. Therefore, we have developed a powerful new experimental-bioinformatic method for correcting bias from PCR-based library preparation of antibody repertoires. Importantly, this method enables highly accurate relative abundances (frequencies) to be determined for antibody clones within a repertoire (T. Khan et al., Manuscript in preparation).
Systems vaccinology
High-throughput immune repertoire analysis offers the promise to aid or replace existing immunological technologies such as screening (e.g. hybridomas recombinant surface display, etc), antibody measurements (e.g. titers), and cellular characterization (e.g. ELISPOT, cell culture supernatant cytokine quantification, and flow cytometry). We are developing systems vaccinology framework for that will facilitate the characterization of immunomodulation by novel means, evaluating alterations to the entire humoral landscape. There is also great promise for utilizing these large data sets to quantitatively characterize the level of polarization of the humoral repertoire post-vaccination. Measurements such as these can be used to screen vaccine formulations to ensure patients are left with not only a strong immune response, but one that enables broad epitope protection. Formulations capable of inducing broad epitope protection would be generally preferred, as they would protect the patient from a greater amount of pathogen escape mutants. We are using various statistical approaches to characterize the degree of skewed clonal distributions post-immunization (e.g. Shannon entropy). These types of analyses will lead to improved strategies for creating better quantitative vaccine response characterization methods. We believe these strategies will be able to aid or replace quantitative serum based epitope mapping characterization, as a single method could be used to evaluate responses to all antigens and pathogens. This work will lead to increased speed and efficiency for profiling of vaccine-induced immune responses.