HD-MEAs contribute to detecting proteinopathies in human neurodegenerative diseases
New 2D human-derived cell-culture model developed and electrophysiologically characterized, which led to discovery of new protein, neuronal pentraxin 2 (NPTX2), playing an important role in neurodegenerative diseases amytrophic lateral sclerosis (ALS) and frontotemporal lobar dementia (FTLS).
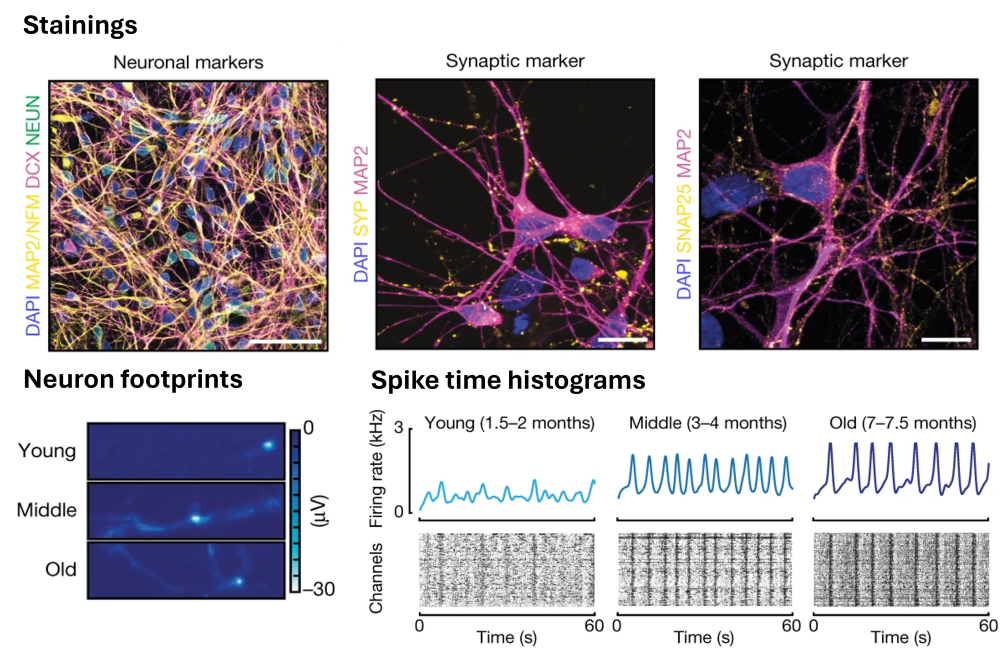

Human cellular models of neurodegeneration require reproducibility and longevity for simulating age-dependent diseases. Such systems are particularly needed for TDP-43 (transactive-response DNA-binding protein 43 kDa) proteinopathies, which involve human-specific mechanisms that cannot be directly studied in animal models. A hyper-phosphorylated, ubiquitinated and cleaved form of TDP-43 - known as pathologic TDP-43 - is a major disease protein in frontotemporal lobar dementia (FTLD) and in amyotrophic lateral sclerosis (ALS). Here, human induced pluripotent stem cell-derived, colony morphology neural stem cells (iCoMoNSCs) were generated via manual selection of neural precursors. Differentiated iCoMoNSCs formed a self-organized multicellular system consisting of synaptically connected and electrophysiologically active neurons, which matured into long-lived functional networks (iNets). Neuronal and glial maturation in iNets was similar to that of cortical organoids. Our group specifically used high-density microelectrode arrays (HD-MEAs) to assess the electrophysiologcal activity of the neuronal networks across scales. The collaborative work was published in Nature (M. Hruska-Plochan, S. Ronchi, et al., "A model of human neural networks reveals NPTX2 pathology in ALS and FTLD", Nature 2024, (DOI: 10.1038/s41586-024-07042-7).
It was found that overexpression of wild-type TDP-43 in a minority of neurons within iNets led to progressive fragmentation and aggregation of the protein, resulting in a partial loss of function and neurotoxicity. The strongest misregulated target encoded the synaptic protein neuronal pentraxin 2 (NPTX2). When NPTX2 was overexpressed in iNets, it exhibited neurotoxicity, whereas correcting NPTX2 misregulation partially rescued neurons from TDP-43-induced neurodegeneration. Notably, NPTX2 was consistently misaccumulated in neurons from patients with ALS and FTLD with TDP-43 pathology. The collaborative work directly links TDP-43 misregulation and NPTX2 accumulation, thereby revealing a TDP-43-dependent pathway of neurotoxicity.