Tissue Systems
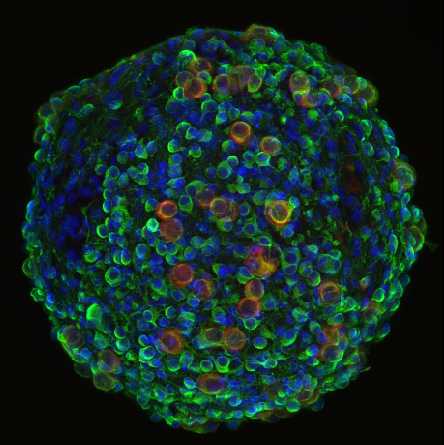
In-vitro cell-based assays play a key role in drug discovery and can provide essential information on the efficacy and toxicity of new compounds. Advanced in-vitro model systems offer the possibility of using human-based cell material in a more physiological context and to replace animal experiments. In order to increase the predictability of such assays, 3D tissue constructs receive more and more attention, as they better recapitulate in-vivo characteristics. We predominantly work with spherical microtissues, produced by the hanging drop technology or formed in ultralow-adhesion well plates, as they are comparably simple and reproducible to fabricate, and feature organotypic functionality and physiology. Their spherical shape and compact constitution make them ideal candidates for handling in microfluidic structures.

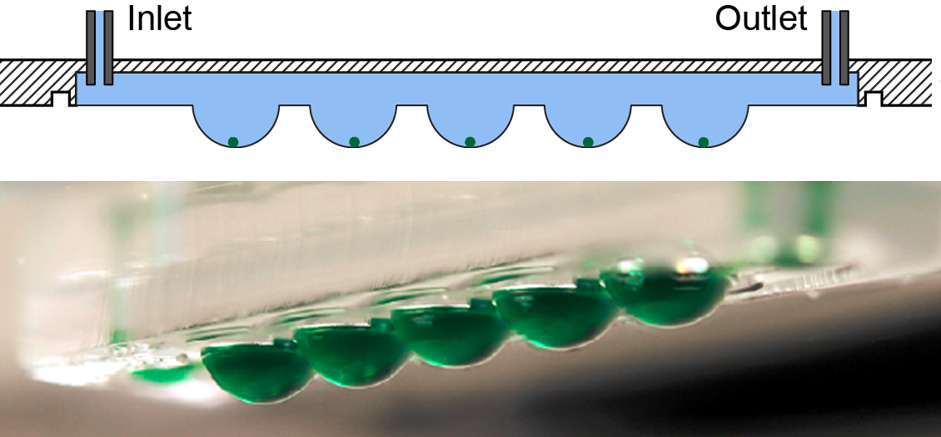
As the microtissues mostly include adherent cells (liver, heart, brain, pancreas etc.), one has to apply methods, where the spheroids do not adhere to surfaces and, thereby, remain intact over extended experimentation times. One possibility is to use the concept of "hanging-drop networks", in which the spheroids are located at the liquid-air-interface at the bottom of the hanging drops. The hanging drops are then interconnected via microfluidic channels to provide perfusion, supply of nutrients, dosage of compounds, or to interconnect them to other tissue types to realize "body-on-a-chip" arrangements.
Alternatively, one can host the spheroids in microtissue compartments in well plates, which are continuously tilted in a rocking-like motion, so that the spheroids constantly perform slight motions and cannot adhere to the surface, which additionallly is coated with non-adhesive films. The tilting motion also ensures perfusion and enables metabolic tissue interaction through the connection channels. Both approaches allow for mimicking conditions of the human body and for predicting the impact of new compounds on multi-organ systems.
Another group effort includes to develop microfluidics-based systems to enable high-resolution imaging of tumor invasion into complex tissue environments over several days. Organotypic slices retain the composition and cytoarchitecture of their tissue of origin, which renders them suitable for investigating tumor-microenvironment interactions. Tissue slices are accommodated in a controlled microfluidic chamber, which allows for continuous optical access with high-numerical-aperture objectives while preserving sterility and tissue viability. We used the platform to investigate medulloblastoma invasion into organotypic cerebellar slices.
Relevant publications
C. Lohasz, T. Häfeli, D. Hasanova, L. Hölting, M. Rudnik, L.-A. Ligeon, S. Lützow, A. Mehta, B. Kritzer, S. Laternser, J. Nazarian, A. Hierlemann, O. Frey, M. Modena, "A microfluidic platform for the co-culturing of microtissues with continuously recirculating suspension cells", Microsystems & Nanoengineering 2025, 11, 184 (DOI:.10.1038/s41378-025-01028-9). external page Online
C. Lohasz, J. Loretan, D. Sterker, E. Görlach, K. Renggli, P. Argast, O. Frey, M. Wiesmann, M. Wartmann, M. Rausch, A. Hierlemann, "A microphysiological cell-culturing system for pharmacokinetic drug exposure and high-resolution imaging of arrays of 3D microtissues", Frontiers in Pharmacology 2021,12:785851 (DOI: 10.3389/fphar.2021.785851). external page Online
O.T. P. Nguyen, P. M. Misun, C. Lohasz, J. Lee, W. Wang, T. Schroeder, A. Hierlemann, "An immunocompetent microphysiological system to simultaneously investigate effects of anti-tumor natural killer cells on tumor and cardiac microtissues", Frontiers in Immunology 2021, Article 781337 (DOI: 10.3389/fimmu.2021.781337). external page Online
P. Misun, B. Yesildag, F. Forschler, A. Neelakandhan, N. Rousset, A. Biernath, A. Hierlemann, O. Frey, "In-vitro platform for studying human insulin release dynamics of single pancreatic islet microtissues at high resolution", Advanced Biosystems 2020, Article 1900291 (DOI: 10.1002/adbi.201900291). external page Online
J. Boos, P. Misun, A. Michlmayr, A. Hierlemann, O. Frey, "Microfluidic multitissue platform for advanced embryotoxicity testing in vitro", Advanced Science 2019, Article 1900294 (DOI: 10.1002/advs.201900294). external page Online