CMOS Microsystems
The notion of external page "microsystem" implies that we do not only devise single transducers, i.e., sensors or actuators, but that we develop rather complex systems comprising transducers, associated analog and digital circuitry units, and addressing and interface units on the same microchip. Many of the systems are realized in Complementary-Metal-Oxide-Semiconductor or external page CMOS technology, which currently is the standard technology for the fabrication of integrated circuits on microelectronic chips. We have a fully established CMOS circuit design environment to perform analog and digital design of complex systems on chips.
Key advantages of microsystem integration include:
Signal Quality
- On-chip signal conditioning can be done close to the signal source, which enables the readout of low-level signals from very small transducers
- Miniaturization is possible without deterioration in transducer and overall system performance
- Analog-to-digital conversion can be done on chip, so that only robust digital signals are transmitted
Connectivity
- On-chip multiplexing enables to acquire signals from thousands of small transducers via few off-chip connections
- Integrated systems offer the possibility to perform massively parallel readout or multi-parameter detection
Microelectrode and Biosensor Systems
Traditional external page microelectrode arrays (MEAs) are circuitless chips carrying arrangements of usually 60 electrodes that are used for multisite extracellular recording from electrogenic cells, such as neurons or heart cells. They are used in the fields of neuroscience and biosensing, to study fundamentals of neuronal information processing, of learning processes, of aging and mental diseases, or to assess the behavior of electrogenic cells upon exposure to pharmacological agents or hazardous substances.
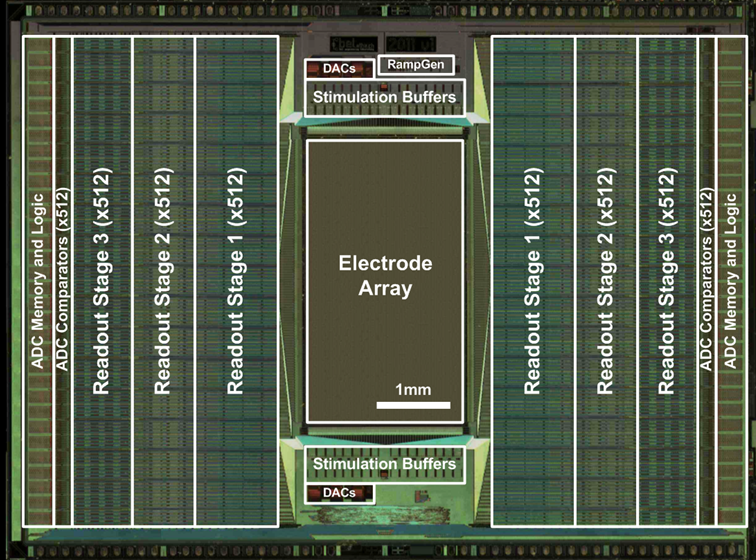
The development and use of CMOS-based devices can overcome limitations of passive MEAs, in particular with respect to performing measurements at high spatial and temporal resolution at excellent signal quality. Through on-chip signal multiplexing, a large number of electrodes can be addressed to achieve a high spatio-temporal resolution in recording and stimulation scenarios. Moreover, weak neuronal signals can be conditioned right at the electrodes by dedicated circuitry units, which provide a large signal-to-noise ratio. Finally, analog-to-digital conversion is performed on chip, so that only stable, digital signals are transmitted.
Several generations of CMOS-based high-density microelectrode arrays (HD-MEAs) have been developed in our group during the past years. We currently work on arrays of thousands of electrodes (up to 60,000) for electrophysiological and impedance recordings at subcellular resolution (13 μm electrode pitch, 5 - 8 μm electrode diameter, 5,500 electrodes per mm²) in active areas between 2 x 2 mm² and 3 x 4 mm². All electrodes are bidirectional and can also be used for stimulation in current and voltage mode.
Moreover, a variety of CMOS-based microsensor systems has been developed in our group. The transducers include microelectrodes for impedance and electrochemical measurements and metallic or semiconducting nanowires. Larger sets of transducers have been combined into arrays and have been monolithically integrated with the needed front-end and signal conditioning circuitry (analog and digital circuitry units).
Relevant publications
M. Schröter, F. Cardes, C. Bui, L. Dodi, T. Gänswein, J. Bartram, L. Sadiraj, P. Hornauer, S. Kumar, M. Pascual-Garcia, and A. Hierlemann, "Advances in large-scale electrophysiology with high-density microelectrode arrays", Lab on a Chip 2025, in press (DOI: 10.1039/D5LC00058K). external page Online
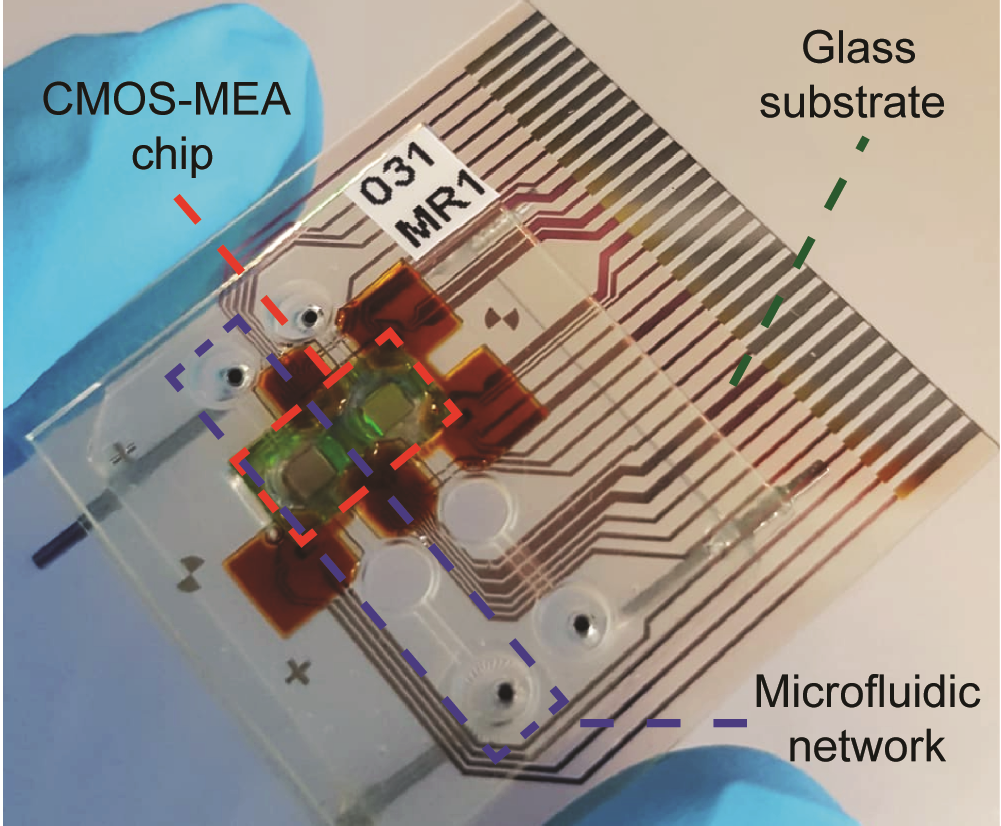
R. Bounik, A. Landolt, J. Lee, V. Viswam, F. Cardes, M. Modena, and A. Hierlemann, "Seamless integration of CMOS microsensors into open microfluidic systems", Lab on a Chip 2025, in press (DOI: 10.1039/D4LC01000K) external page Online
F. Cardes, E. Azizi, A. Hierlemann, "A time-domain readout technique for neural interfaces based on VCO-timestamping", IEEE Transactions on Biomedical Circuits and Systems 2023, 17 (3), p. 574-584 (DOI: 10.1109/TBCAS.2023.3274834). external page Online
X. Yuan, M. Schröter, M. Engelene J. Obien, M. Fiscella, W. Gong, T. Kikuchi, A. Odawara, S. Noji, I. Suzuki, J. Takahashi, A. Hierlemann, U. Frey, "Versatile live-cell activity analysis platform for characterization of neuronal dynamics at single-cell and network level", Nature Communications 2020, 11, 4854 (DOI: 10.1038/s41467-020-18620-4). external page Online
J. Dragas, V. Viswam, A. Shadmani, Y. Chen, R. Bounik, A. Stettler, M. Radivojevic, S. Geissler, M. Engelene J. Obien, J. Müller, A. Hierlemann, "In-vitro multi-functional microelectrode array featuring 59760 electrodes, 2048 electrophysiology channels, stimulation, impedance measurement and neurotransmitter detection channels", IEEE Journal of Solid-State Circuits, 2017, Volume 52 (6), pp. 1576-1590 (DOI:10.1109/JSSC.2017.2686580). external page Online