Biosystems engineering
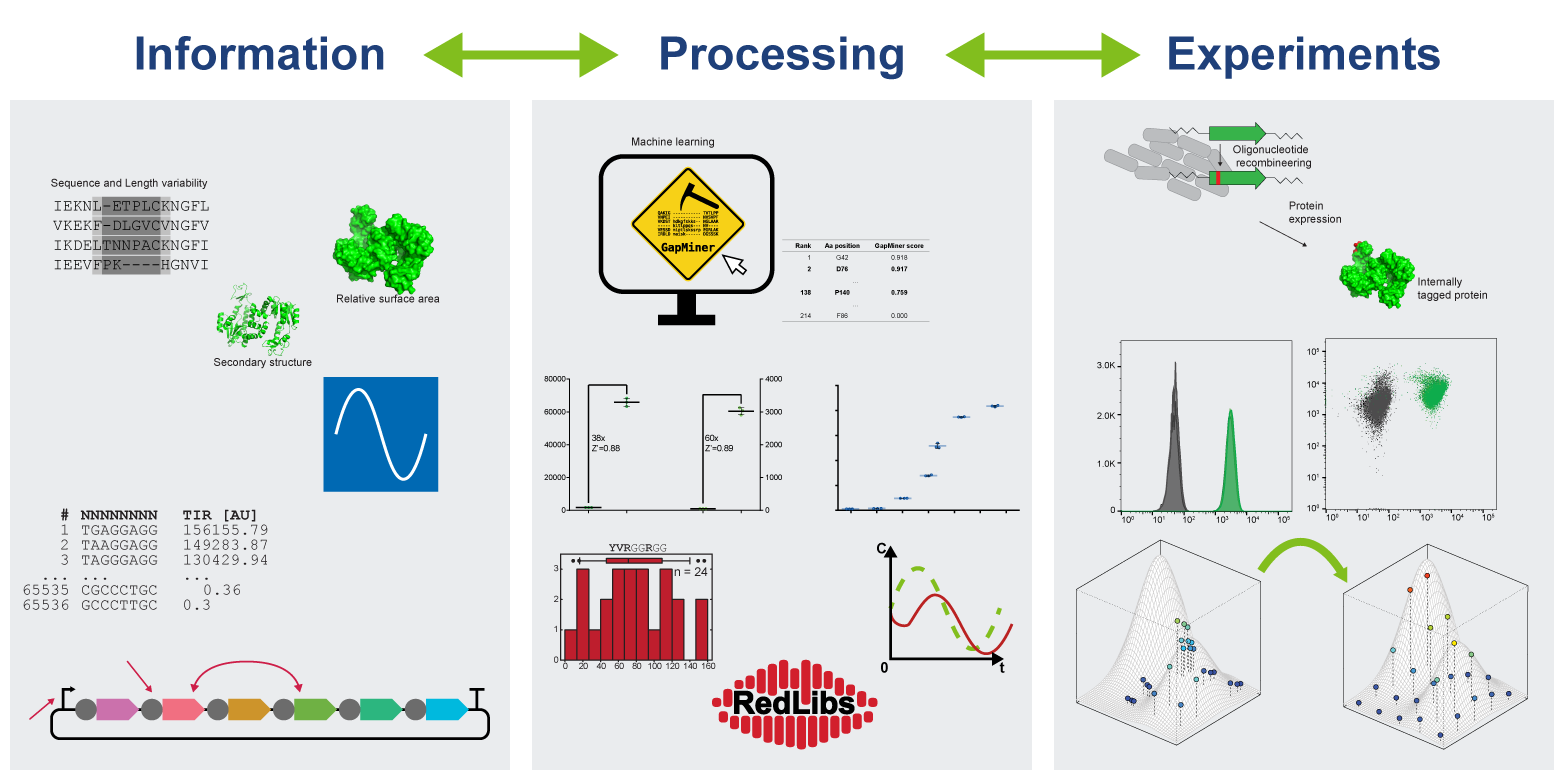
The transformation of bioengineering towards a true engineering discipline is one of the major pursuits in the field of synthetic biology. Gaining control of biological systems ultimately requires the translation of quantitative understanding into applied methods in order to construct functional devices from biological parts. These approaches can be applied from the single protein level to synthetic biosensor circuits and up to large multiprotein systems.
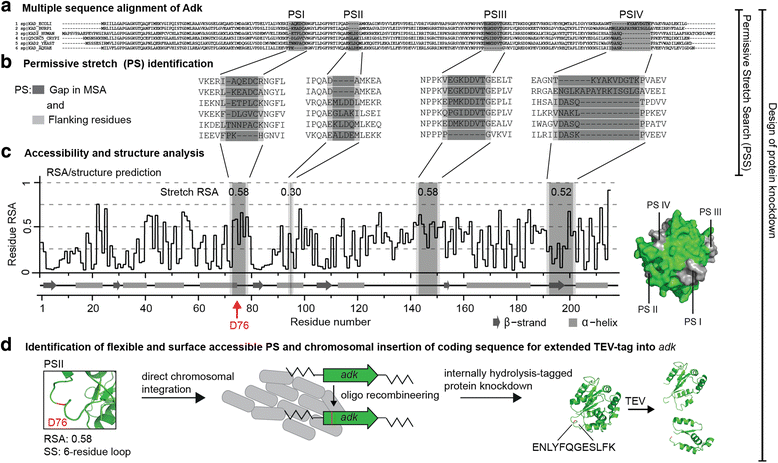
Besides random and semi-rational approaches of protein engineering that require high-throughput experimenting methods to select for a desired function, we are developing design principles to forward-engineer new functions into proteins. One of these functions is controlled deactivation or “off-switching”. To design “switchable proteins”, we developed a workflow called permissive stretch search to identify internal tagging sites in proteins based on primary sequence information only.
References
S. Oesterle, T.M. Roberts, L.A. Widmer, H. Mustafa, S. Panke and S. Billerbeck: Sequence-based prediction of permissive stretches for internal protein tagging and knockdown, BMC Biology, 2017, external page DOI.
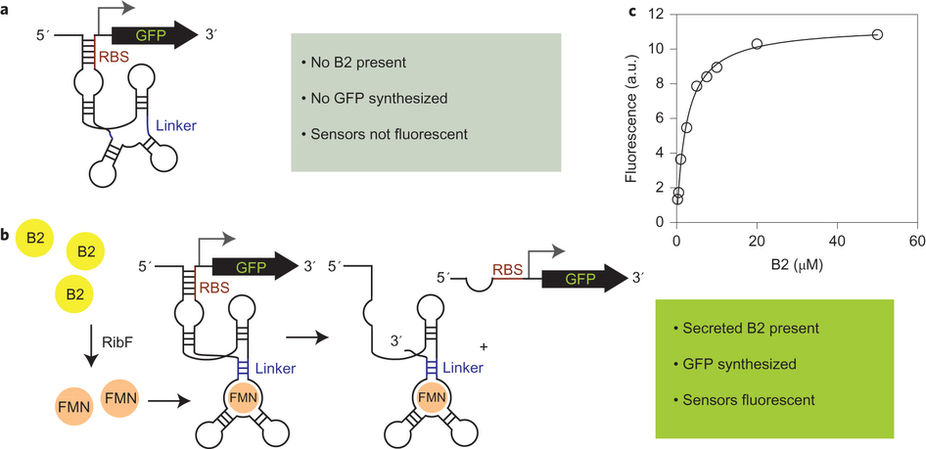
Biosensor circuits can be broken down to blueprints of functional units similar to the electronic parts known from traditional sensor schemes: input recognition, signal transduction, and output. While the corresponding genetic parts are encodeable in a relatively straight-forward manner, the challenge lies in fine-tuning the behavior of the system. In order to generate a useful output signal, the sensitivity of the system needs to be high enough to discriminate between similar, but increased, product titers or diagnostic marker concentrations. Furthermore, the dynamic range has to be large enough to allow for enrichment based on increased output, and the linear portion of the sensor-response should cover the whole titer concentration range. Such fine-tuning efforts include, for instance, changing input recognition by means of directed evolution, as demonstrated for riboflavin/FMN recognition.
References
A. Meyer, R. Pellaux, S. Potot, K. Becker, H.-P. Hohmann, S. Panke and M. Held: Optimization of a whole-cell biocatalyst by employing genetically encoded product sensors inside nanolitre reactors, Nature Chemistry, 2015, external page DOI.
Maximizing the information and control of the constituents of a multiprotein system allows for efficient optimization of synthetic pathways producing value-added compounds. A particularly high level of control can be achieved when employing in vitro systems. With precise dosing in a time-dependent manner and quantitative real-time analysis, we are able to fine-tune a complex reaction system and even predict optimal conditions.
Additionally, our laboratory is interested in extending this information-driven optimization process to in vivo multiprotein systems. To this end, we design smart libraries that allow us to probe a maximal expression level space with minimal experimental effort either on a plasmid or directly on the genome. During the optimization process, we gain additional information about the behavior of the system with respect to different expression level combinations and thus allow us to further optimize our systems of interest.
References
M. Bujara, M. Schümperli, R. Pellaux, M. Heinemann and S. Panke: Optimization of a blueprint for in vitro glycolysis by metabolic real-time analysis, Nature Chemical Biology, 2011, external page DOI.
C. Hold, S. Billerbeck and S. Panke: Forward design of a complex enzyme cascade reaction, Nature Communications, 2016, external page DOI.
M. Jeschek, D. Gerngross and S. Panke: Rationally reduced libraries for combinatorial pathway optimization minimizing experimental effort, Nature Communications, 2016, external page DOI.
S. Oesterle, D. Gerngross, S. Schmitt, T.M. Roberts and S. Panke: Efficient engineering of chromosomal ribosome binding site libraries in mismatch repair proficient Escherichia coli, Scientific Reports, 2017, external page DOI.