Research
Novel Photoenzymes
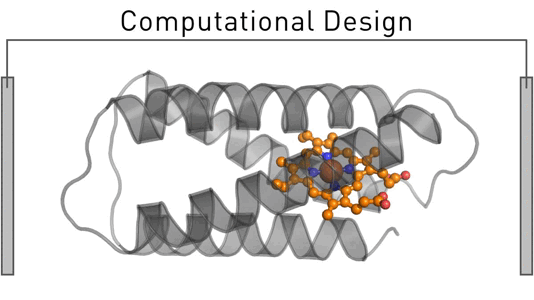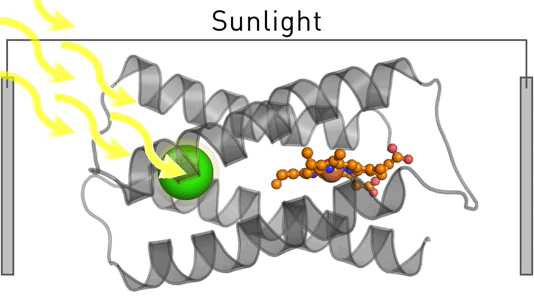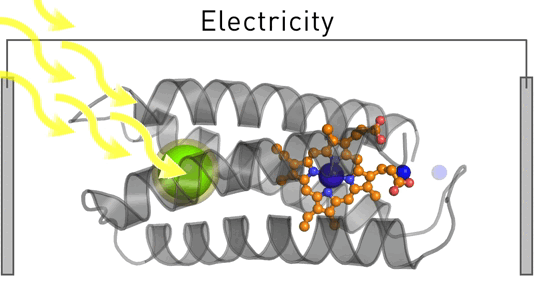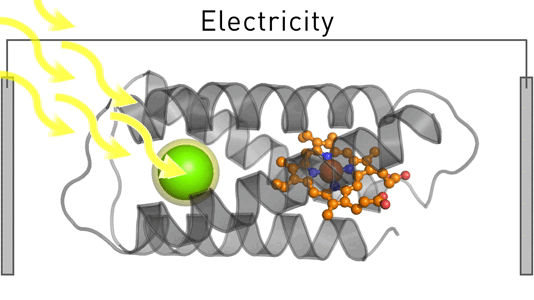
Satisfying humankind's energy demand from sustainable sources is a critical challenge in the 21st century. To address this challenge, we employ design and evolution to create novel photo-biocatalysts by equipping existing enzymes with binding sites for affordable organic photosensitizers. We have recently created a photoenzyme with photovoltaic activity that we are currently incorporating into biohybrid solar cells. Our approach can be generally employed to induce photocatalytic activity – and we use it to create sustainable biocatalysts that target critical societal challenges such as renewable energies, carbon capture, food security, and solar fuels.
Creating Enzymes by Design and Evolution

We combine computational design and directed evolution to create novel enzymes. Specifically, we use design and evolution to tailor artificial photocatalytic enzymes that harness solar energy for biocatalysis and energy production. Furthermore, we use experimental and computational methods to understand how enzymes work by dissecting how design and evolution give rise to catalytic activity.
- external page call_made Designing better enzymes: Insights from directed evolution. Curr Opin Struct Biol 2021.
- external page call_made Emergence of a negative activation heat capacity during evolution of a computationally designed enzyme. J Am Chem Soc 2019.
- external page call_made Efficient Lewis acid catalysis of an abiological reaction in a de novo protein scaffold. Nat Chem 2021.
- external page call_made Speeding up enzyme discovery and engineering with ultrahigh-throughput methods. Curr Opin Struct Biol 2018.
Antimicrobrial Resistance
As antimicrobial resistance increases globally, infections with resistant bacteria pose a major public health threat. In our current work, we study the emergence of new resistances through the lens of directed evolution. Our work aims to aid the creation of novel antibiotics less prone to resistance and guide personalized health care.
How Enzymes Work
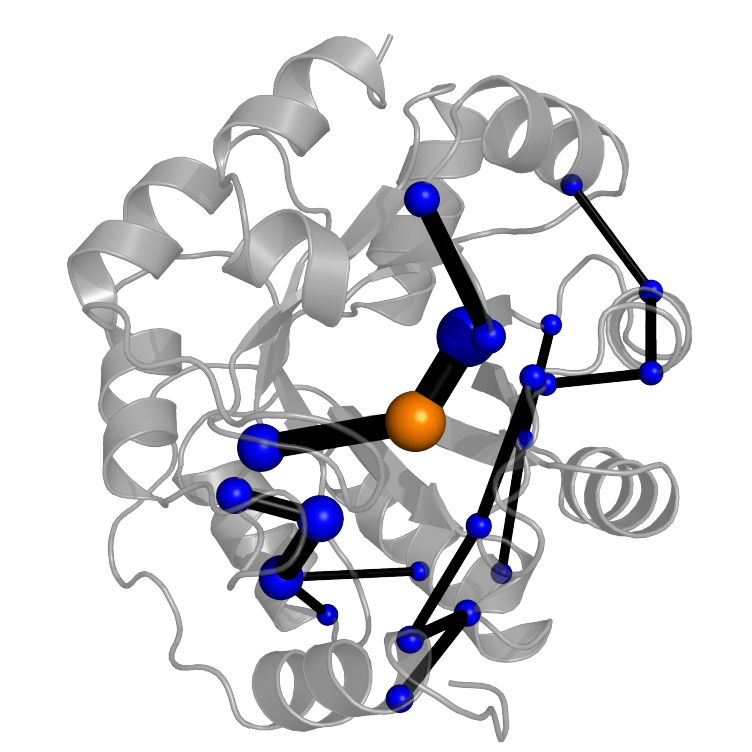
Enzymes can accelerate reactions with unequaled efficiency – understanding how enzymes work has been a longstanding goal in biochemistry. Comprehensive insights into the origins of catalysis will foster the creation of new biocatalysts for application. Directed evolution of designer enzymes can provide unparalleled insights into how enzymes work by revealing how catalytic activity emerges during evolution. To that end, we have created an enzyme accelerating its reaction by almost eight orders of magnitude using directed evolution. Evolution sometimes introduces new catalytic residues and complex catalytic motifs to boost catalysis. Notably, evolution not only introduces catalytic residues – but also stabilizes catalytically superior conformations to boots activity. These effects are not restricted to the active site. We showed that enzymes can recruit the whole protein for catalysis using dynamical networks that selectively recognize the transition state . Together, our insights suggest novel strategies to engineer more efficient enzymes for application.
- external page call_made Evolution of dynamical networks enhances catalysis in a designer enzyme. Nat Chem 2021.
- external page call_made Rigidifying a de novo enzyme increases activity and induces a negative activation heat capacity. ACS Catal 2021.
- external page call_made Beneficial substrate partitioning boosts non-aqueous catalysis in de novo enzyme-alginate beads. bioRxiv 2021.
- external page call_made How directed evolution reshapes the energy landscape in an enzyme to boost catalysis. Science 2020.
- external page call_made Contribution of Oxyanion Stabilization to Kemp Eliminase Efficiency. ACS Catal 2020.