Super-resolution Microscopes
The SCF houses several super-resolution (SR) systems. Pushing technology to its limit, these high-end microscopes provide best-in-class resolution beyond the traditional diffraction barrier for a variety of imaging scenarios.
Traditional light microscopy is limited to a resolution of about 220nm under practical lab conditions - the so-called the diffraction barrier. New SR technologies use an intelligent mix of applied mathematics, physics and chemistry to overcome this limitation.
Our super-resolution microscopes can be classified into three types of technologies achieving different levels of resolution: resolution-enhancing modules (ca. 120 nm), stimulated emission depletion microscopy (STED, ca. 50nm) and localization-based SR microscopy (ca. 2nm). The different instruments require dedicated sample preparation protocols and may only aply to certain types of samples. Some of these microscopes also provide superresolution in 3D.
For detailed descriptions of the individual workstations (all specifications, filter settings, etc.) and interactive spectra viewers, see our internal Wiki (D-BSSE login required).
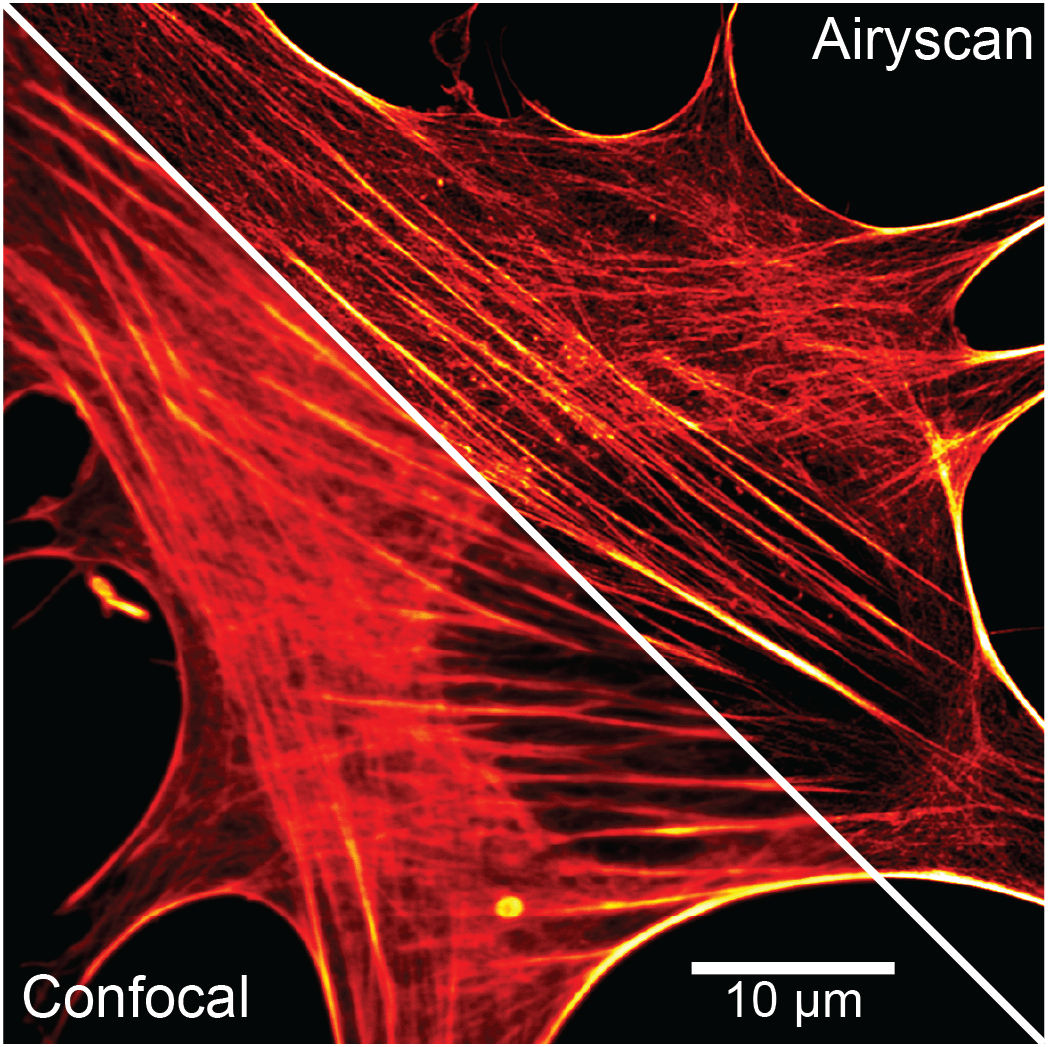
The first class can be described as resolution-enhancing SR modules of existing setups. The best resolution of these systems ranges around 120-140 nm.The following systems fall in this category:
- Airyscan 2 on point-scanning Zeiss LSM980 confocal multiphoton microscope - Honeycomb array detector which helps to improve resolution up to 2-fold beyond the diffraction limit, based on optical re-assignment in post-processing.
- SoRa module on W1-SoRa Spinning Disk confocal microscope - Special spinning disk module which applies optical re-assignment during acquisition, providing an improved resolution of up to 2-fold beyond the diffraction limit.
- SRRF module on Nikon WF1wide field microscope - Super-Resolution Radial Fluctuations (SRRF) imaging using a dedicated camera module that analyzes radial and temporal fluorescence intensity fluctuations in an image sequence to generate a super‑resolution image, providing variable resolution improvement of 2-6x beyond the diffraction limit.
The second class of super-resolution methods involves spatially selective deactivation of fluorophores in samples using a so-called 'donut beam'. In STimulated Emission Depletion (STED) microscopy, one minimizes the volume of fluorescence emission at the focal point, thus enhancing the achievable resolution. Depending on settings and labeling, a resolution of up to ~50nm could be achieved. Our in-house setup is the following:
Aberrior STEDYCON
The Abberior STEDYCON setup combines a super-intuitive acquisition software with a compact but capable 2D STED system capable of achieving down to 30 nm resolution (external page specific dyes required). With only a few clicks needed between a sample overview and the first STED image, this system offers the easiest transition from a point-scanner confocal into the super-resolution world.
- Microscope base: Zeiss Axio Observer Z1 (inverted)
- Available excitation lasers: 405, 488, 561 and 640 nm
- Available STED lasers: 775 nm
- Detectors: 3 filter-based APDs capable of photon counting and gated detection
- Objectives: 100x
The last family of super-resolution methods at SCF, Single-Molecule Localization Microscopy (SMLM) can dramatically improve spatial resolution over standard diffraction-limited imaging techniques, with localization precision in the single nm range. To achieve this, the emission of distinct molecules is separated over time (through photoswitching, photoactivation or photoconversion), allowing the localization of individual molecules. The SCF is equipped with a MINFLUX Single Molecule Localization Microscope capable of achieving 1-3 nm localization precision in 3D and ultra-fast single-molecule tracking up to 10 kHz.
Abberior MINFLUX (MINFLUX)
The Abberior MINFLUX microscope defines an entirely new class of super-resolution methods that combines the strengths of the single molecule localization family and STED microscopy. Individually activated fluorescence-emitting molecules are localized through an iterative scanning approach using an excitation beam with a dark center, which reduces the number of emitted photons required per localization.
MINFLUX can routinely achieve single-digit nm localization precision along x, y and z axis. Thanks to its photon efficiency, multiple localizations can be generated during a single emission event. This, together with the variety of metrics acquired during the localization process, enables post-processing statistical analysis and filtering.
The instrument can operate in two modalities:
- Localization mode: Either 2D or 3D, the emitters in the field of view are localized sequentially one by one, until the underlying structure is reconstructed.
- Tracking mode: A single fluorophore is tracked in 3D over time with a frequency up to 10 kHz yielding the track of the movement of this molecule with an extraordinary high spatial and temporal resolution.
Setup:
- Microscope base: Olympus IX83 (inverted)
- Available lasers (point-scanner confocal mode): 405, 488, 561 and 640 nm
- Available lasers (MINFLUX mode): 561 and 640 nm
- Detectors: 5 filter-based APDs. 2-Color MINFLUX can be performed for some compatible dye pairs
- Active sample stabilization system with sub-nm precision
- Objectives: 20x, 40x and 100x oil immersion (for MINFLUX mode)
More information on the manufacturer website external page here.