Unravelling the secrets of viral DNA binding
A study led by researchers from the group of SNF Ambizione Fellow Nicolas Huguenin-Dezot has found that a protein forms a chain along single-stranded DNA, aiding a bacteriophage in copying its genetic material. Using advanced structural biology, biochemical, and bioinformatics techniques, the team uncovered a previously unknown protein fold binding to DNA in a unique way. This discovery could inspire innovations in medicine and biotechnology.
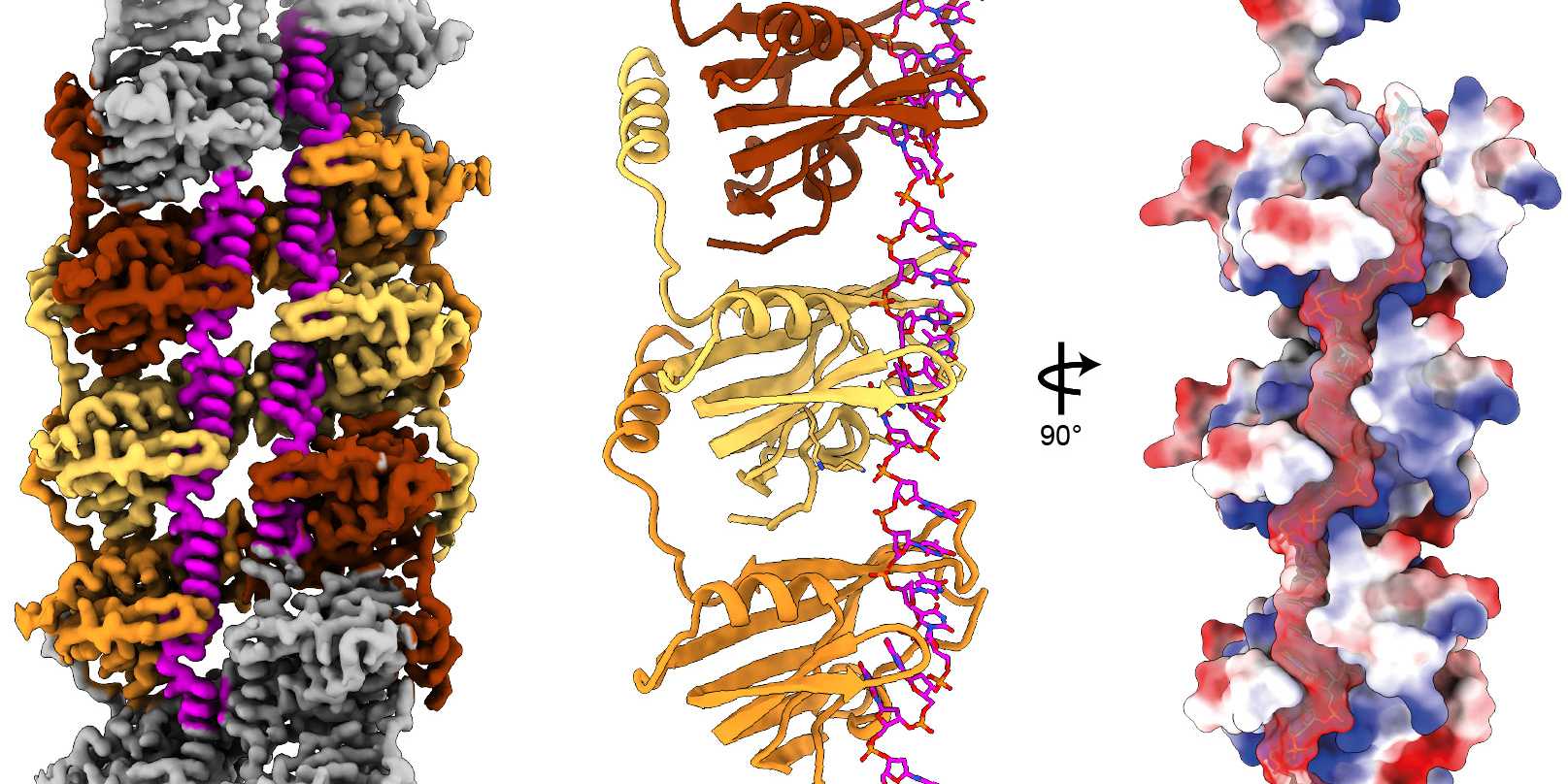
Viruses that infect bacteria, known as bacteriophages, have evolved highly sophisticated ways to interact with genetic material. One particularly intriguing mechanism is protein-primed DNA replication, which certain viruses, including the so-called bacteriophage PRD1, use to replicate their DNA. Protein-primed DNA replication involves specialised proteins that help stabilise the DNA strands during replication. In PRD1, a key player in this process is the P12 protein, which exhibits remarkable properties.
Using advanced imaging techniques such as cryo-electron microscopy (cryo-EM), researchers from the group of SNF Ambizione Fellow Nicolas Huguenin-Dezot and colleagues at the University of Basel discovered that P12 binds to single-stranded DNA (ssDNA), forming a chain-like structure along the DNA strand. The study, which just appeared in Nucleic Acids Research, reveals that a flexible tail domain on P12 acts as an anchor, allowing each P12 protein to interact with a neighbouring protein, thereby stabilising the assembly into a continuous chain. Such ssDNA–protein complexes are rarely observed, and the published structure is the first of its kind.
To complement these structural findings, the team conducted extensive biochemical experiments, uncovering new molecular details of how P12 interacts with ssDNA. Additionally, detailed bioinformatic analyses revealed that P12 adopts a previously unidentified protein fold, which the researchers positioned within the broader landscape of so-called ssDNA-binding proteins (SSBs). Since SSBs play crucial roles in DNA replication, repair, recombination, and transcription, understanding how they function provides key insights into these essential biological processes.
Beyond their fundamental significance, bacteriophages like PRD1 offer exciting opportunities for biotechnology. By shedding light on how PRD1 interacts with DNA, this research paves the way for innovative applications in science and medicine. These findings could contribute to improving gene delivery systems for medical therapies, advancing novel biotechnological tools, or even guiding the development of antiviral strategies.
Acknowledgement
We thank Professor Sven Panke for helpful discussions and sharing the group facilities. We are grateful to P. Spies for his help with titration experiments. We kindly acknowledge P. Afanasyev (ScopeM) and the BioEM Lab of the Biozentrum, University of Basel, for their support. We also thank Professor Torsten Schwede, the members of the Schwede lab and sciCORE at the University of Basel for providing computational resources and support.
Find journal article published in Nucleic Acids Research
Lena K Träger, Morris Degen, Joana Pereira, Janani Durairaj, Raphael Dias Teixeira, Sebastian Hiller, Nicolas Huguenin-Dezot (2025) external page Structural basis for cooperative ssDNA binding by bacteriophage protein filament P12. Nucleic Acids Research, Volume 53, Issue 5, 24 March 2025, gkaf132, https://doi.org/10.1093/nar/gkaf132
Learn about research in the group led by Nicolas Huguenin-Dezot.